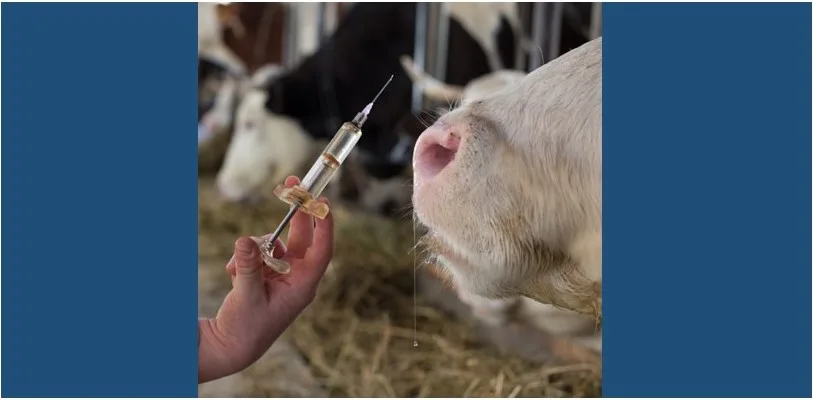
BROOKINGS, S.D. – Medgene Labs, a South Dakota based animal health company, has begun a field trial on their H5N1 vaccine in cattle. The trial began in October at a contract research facility. The goal of the field trial is to demonstrate a reasonable expectation of efficacy and preliminary safety in animals specific to the company’s H5N1 vaccine, data to support a conditional license by the U.S. Department of Agriculture.
Medgene has successfully produced prescription platform vaccines for swine, cattle, rabbit and deer industries in the U.S. and internationally. Chief Operating Officer Tom Halbur expressed optimism, stating that their rapid-response technology is a benefit for the USDA Center for Veterinary Biologics (CVB) and the dairy industry.
The CVB Notice permits vaccine studies to be conducted outside of containment facilities, which could speed up the licensure of H5N1 vaccines for dairy cows. Medgene expects to receive conditional or full licensure from the CVB to produce and distribute the H5N1 vaccine once it is proven safe and effective in dairy cattle.
Dr. Alan Young, co-founder and Chief Technology Officer for Medgene stated, “As unfortunate as the H5N1 spread is, this is exactly the kind of situation that our technology was created to address. Our goal from the very beginning of this outbreak was to be ready for our cattle customers whenever the USDA gave us the green light.”
According to a USDA spokesperson, “USDA has authorized the acceptance of licensure applications for an initial field study, under specific conditions, of nonviable, non-replicating vaccine against HPAI-H5 to be administered to dairy cattle to evaluate safety, as one part of several USDA vaccine licensure requirements. Multiple vaccine manufacturers have expressed interest in beginning field safety trials. This is just one of several steps in achieving vaccine licensure.”
Medgene’s H5N1 cattle vaccine study is expected to be complete in five weeks, with results then shared with the USDA